How to make the most of Crash Courses
A crash course is an intensive and condensed learning program designed to cover a significant amount of material in a short period. These courses aim to provide students with a focused review of key concepts, important theories, and essential problem-solving techniques. Often conducted over a few days or weeks, crash courses are particularly popular among students who are seeking a last-minute boost in their exam preparation.
What To Expect At Learners’ Lodge Crash Courses:
Focused Learning
Our crash course provides targeted learning to help clarify concepts and fill gaps in your understanding.
Make The Most of Time
Time is a limited resource during exam preparations, and crash courses are designed to make the most of it. We condense material into a shorter timeframe which provides a structured study plan that can keep you on track.
Refresh Topics
Refresh topics during our targeted crash courses. This can enhance your memory recall during the exam, making it easier to retrieve the relevant information.
Exam Strategy
We give guidance on exam strategies, such as time management, question prioritization, and effective study techniques to help you in your exam environment.
How to make the most of Crash Courses:
Here are some tips to ensure you get the most out of your crash course experience:
Prioritize Topics
Identify the key topics or concepts you’re struggling with the most and focus on those during the crash course. Prioritizing areas of weakness can yield significant improvements in your overall understanding.
Active Engagement
Actively participate in the course by asking questions, solving problems, and engaging in discussions. The more you interact with the material, the better you’ll retain it.
Supplemental Study
Treat the crash course as a supplement to your prior studies rather than a replacement. Review your class notes and textbooks before and after the course to reinforce your understanding.
Practice, Practice, Practice
While crash courses provide insights and knowledge, practice remains crucial. Solve past exam papers and sample questions to apply what you’ve learned and gain familiarity with the exam format.
As the examination season approaches, the atmosphere in junior colleges becomes charged with anticipation and a sense of urgency. Use our crash courses to get ahead!
Ace GP (General Paper) subject in JC
The General Paper (GP) plays an important role in the GCE A Levels, as it is a subject with profound benefits that extend beyond secondary school. Mastering GP is not only a requirement for success in the A level examination but a worthwhile general goal which students should pursue. This is because the subject is intended to hone candidates’ ability to think critically, communicate effectively, and engage in a discussion of current affairs; all of which are skills that are essential in tertiary education, in the workplace, and more generally, in life.
Here are some tips on how to ace your GP (General Paper) subject in JC:
Make the Most of Accessible Materials
Singaporean students should access local news outlets like The Straits Times, which offer insights into current affairs and societal issues. By reading and analyzing articles from these sources, students can strengthen their language skills and gain relevant examples for their essays. Similarly, accessing reputable global news sources provides students with a broader understanding of international events and issues, thereby allowing them to contextualize their arguments within a global framework.


Be Aware of and Proficient at Discussing Local and Global Issues
Singapore’s multicultural environment serves as an excellent context for students to explore topics related to diversity, social cohesion, and cultural awareness in their GP essays. Simultaneously, global knowledge can be incorporated to provide context and demonstrate the broader implications of the issues discussed.
Develop Critical Thinking Ability
GP students will be presented with questions related to issues such as sustainable economic development, environmental protection, and wealth disparity. In order to discuss these issues meaningfully, students must understand what critical thinking entails. It is recommended that sufficient time be allocated to reading commentaries and opinion pieces in news sources, and to analyzing how arguments are presented and countered.


Get Focused Practice
Given the complexity and diversity of questions that can be tested in GP, consistent practice is essential for students to stand out and excel in their examinations. Regular essay writing and comprehension practice enables students to develop speed and accuracy in answering questions. More importantly, there is a need for candidates to identify areas of weakness and commit time and effort to addressing these areas. Through targeted practice, candidates’ general proficiency in the subject will improve.
Why Learners’ Lodge
Our tutors utilize effective strategies that have been developed over many years of teaching the subject. Each of our GP tutors also approaches the teaching of the subject in somewhat different ways so as to cater to the needs of different students.
Our experienced tutors are dedicated to providing invaluable guidance to help students approach GP with confidence and proficiency.
For those seeking GP tuition in Singapore, whether you choose 1-to-1 or small group classes, we are wholeheartedly committed to helping you achieve better grades in GP. For more information on our tuition services, please do not hesitate to contact us.
Let us work together towards excellence in GP!
How to Choose Your Subject Combination
As most students already know, you are bound to face many struggles in your JC education. The first struggle you may face is choosing a subject combination. Choosing a suitable subject combination that plays to your advantage is important because you will not be able to change the subjects that you take. There were students who failed their examinations on purpose in order to retain and re-select their subject combination. However, those who did not want to retain had to force themselves to study for the subjects that they no longer liked, thus compromising their A-Level results. If you are facing trouble finding a suitable subject combination, fret not! We are here to help.
For students who already know what they want to do in the future, awesome! However, do check out your preferred university’s website to double-check if your subject combination meets the requirement. Here are the subject combinations for some of the university courses that are more popular among students:
- Medicine/ Dentistry: H2 Chemistry, H2 Biology/Physics
- Law: Any combination as long as you have GP/KI
- Business: Any combination with at least H1 Mathematics
- Pharmaceutical Sciences/ Pharmacy: H2 Chemistry, H2 Biology/Physics/Mathematics
- Computer Science: H2 Mathematics/ Further Maths/ Physics
- Public Policy and Global Affairs: Any combination as long as you have GP/KI
For students who have no idea about what they want to do in the future, think about whether you prefer the humanities or the sciences, and choose your subject combination based on that. You can also think about your secondary school subject combination. If you took Triple Science and deeply enjoyed the syllabus, you may want to choose a subject combination in the science stream. You may also want to refrain from choosing too many content-heavy subjects for the sake of your sanity. Alternatively, if you like both the humanities and the sciences, you may want to consider a hybrid subject combination where you pick two science subjects and two humanities subjects. However, do check if your school offers the particular subject combination that you are interested in. Regardless of whatever you choose, it will be highly advisable to choose H2 Chemistry as one of your subjects as most university courses (especially science courses), require H2 Chemistry. In addition, if you are still clueless about which subjects to choose, attend the taster classes if your school offers them. Pay attention during these classes and see for yourself if the content interests you.
When in doubt, you can always refer to the SEAB website to find out more information about the syllabus for the respective subjects, or search online for free notes and past year papers, and gauge for yourself if you are able to handle the workload. You can also talk to any of our friendly teachers at Learners’ Lodge and seek their advice!
H1 or H2?
This is another worry that many students face because they are unsure if they can cope with the workload of studying 4 H2s. Don’t worry, you can always take 4 H2 subjects first before dropping one of the subjects to H1 level at the end of J1 or during J2. It will be wise to study 4 H2 subjects first and use your performance in JC examinations to decide which subject to drop to H1 level if you are only keen on taking 3 H2s.
Curious about H1 vs H2 Math specifically? Learn about the difference between H1 and H2 Math at JC Level and how to choose in our short handy guide.
For those who are curious about H3, you will only be able to take H3 subjects at the end of J1, with your examination results taken into consideration.
The Dilemma: Interests vs Ability
The short answer to this dilemma would be to consider the subjects that you are interested in first because you are more likely to stay motivated and study the subjects that you genuinely like.
Although choosing subjects that you are interested in will keep you motivated, some students may start losing interest in the subjects that they once liked if they are constantly unable to do well. Furthermore, being able to score good grades is also important as you need high enough grades to enter university. Good grades are also even more crucial to students who are wishing to enrol in a more competitive course. That being said, being able to score for a certain subject in secondary school does not necessarily mean you will do the same in JC as the rigour of A-Level subjects is greater. Thus, it is not advisable to choose your subject combination only based on the subjects you can do well in, especially if you do not have much interest in them. If possible, try to choose the subjects that you are genuinely interested in. Even if you are unable to excel in those subjects, you will still be enjoying the learning process at the very least.
Why is it important to select the right subject combination in JC
Selecting the right subject combination in Junior College (JC) is important for several reasons:
- Academic Requirements: Different universities and courses have specific subject prerequisites. Choosing the right combination of subjects ensures that you meet the entry requirements for the higher education institutions and courses you are interested in pursuing. Certain subjects may be mandatory or highly recommended for specific fields of study.
- Future Career Options: The subject combination you choose can have an impact on your future career options. Some professions require specific subject knowledge or skills. For example, if you are interested in pursuing a career in medicine, taking science subjects like Biology and Chemistry would be essential. Researching the requirements of the career paths you are considering can help you make informed decisions about your subject choices.
- Personal Interest and Strengths: It is important to select subjects that align with your interests and strengths. When you enjoy studying a subject, you are more likely to put in the effort and perform well. Choosing subjects that you excel in can also boost your overall academic performance, which can have positive effects on your future opportunities and university applications.
- Holistic Development: A well-rounded subject combination can contribute to your holistic development. Combining subjects from different domains, such as Sciences, Humanities, and Arts, can provide you with a broader perspective and a more diverse skill set. This can enhance your critical thinking, problem-solving abilities, creativity, and communication skills, which are valuable in various aspects of life and future career paths.
- Flexibility and Backup Options: Life is dynamic, and your interests and goals may change over time. Selecting a combination that offers flexibility can be beneficial. Having a range of subjects can provide you with backup options if you decide to switch career paths or if your original plans change. It allows you to explore different fields of study before committing to a specific one.
Ultimately, the right subject combination will depend on your individual interests, strengths, future goals, and the requirements of the universities or careers you are aiming for. It’s important to seek guidance from your school counselors, teachers, and mentors who can provide you with personalized advice based on your circumstances and aspirations.
Ace Your Economics Essay
Introduction
Economics essays provide a platform for students to analyze and evaluate complex economic concepts, theories, and real-world scenarios. A well-crafted economics essay showcases a student’s understanding of economic principles, critical thinking skills, and ability to communicate ideas effectively. To ace your economics essay, it is essential to master the art of clear and concise writing, develop a strong argument, and support it with solid evidence. This essay will provide valuable tips and strategies to help you excel in your economics essay writing.
- Understand the Question: The first step in acing an economics essay is to fully comprehend the question or prompt. Take the time to carefully read and analyze the question, identifying the key terms, concepts, and requirements. Pay attention to any specific instructions provided, such as word limits or required sources. Understanding the question will help you structure your essay effectively and ensure that you stay focused on the main topic.
- Plan Your Essay: Once you have a clear understanding of the question, create an outline or plan for your essay. Planning allows you to organize your thoughts and arguments coherently. Consider the main points you want to address, the order in which you will present them, and the evidence or examples you will use to support your arguments. A well-structured essay with a logical flow of ideas will make a stronger impact on the reader.
- Conduct Thorough Research: Economics essays often require supporting evidence and data to strengthen your arguments. Conduct thorough research using reputable sources such as academic journals, books, and reliable websites. Ensure that the sources you use are up-to-date and relevant to your topic. Incorporating data, statistics, and real-world examples will enhance the credibility of your essay and demonstrate a deeper understanding of the subject matter.
- Develop a Strong Thesis Statement: A strong thesis statement is the backbone of an economics essay. It should clearly state your main argument or position on the topic. Your thesis statement should be concise, specific, and arguable. It provides a roadmap for your essay, guiding the reader through your arguments and analysis. Spend time refining your thesis statement to ensure it accurately reflects your main ideas and sets the tone for the rest of your essay.
- Use Clear and Concise Language: Economics essays should be written in clear and concise language. Avoid excessive jargon or technical terms that may confuse the reader. Instead, strive for clarity and simplicity. Present your ideas and arguments in a straightforward manner, using appropriate economic terminology where necessary. Ensure that your sentences are well-structured and that your paragraphs flow logically.
- Analyze and Evaluate: An essential aspect of economics essays is the ability to analyze and evaluate economic theories, policies, or scenarios. Provide a critical analysis of the topic, identifying strengths, weaknesses, and potential implications. Consider alternative viewpoints and counterarguments to strengthen your own arguments. Avoid making unsupported assertions and back up your claims with solid evidence and logical reasoning.
- Use Visuals Effectively: Visual aids such as graphs, charts, or tables can enhance the clarity and impact of your essay. When appropriate, incorporate visuals to illustrate economic trends, patterns, or relationships. Ensure that the visuals are labeled correctly and referenced in the text. Visual representations can provide a concise and visual understanding of complex economic concepts, making your essay more engaging and informative.
- Revise and Edit: Before submitting your economics essay, allocate time for revising and editing. Review your essay for clarity, coherence, and logical flow. Check for grammar, punctuation, and spelling errors. Ensure that your arguments are well-supported and your evidence is properly cited. Consider seeking feedback from peers, professors, or writing centers to gain different perspectives and identify areas for improvement.
Conclusion
In conclusion, writing an economics essay requires careful planning, research, and analysis. By following the tips and strategies outlined above, you can effectively communicate your understanding of economic concepts and theories and present a well-crafted argument. Remember to understand the question, develop a strong thesis statement, conduct thorough research, use clear and concise language, analyze and evaluate, use visuals effectively, and revise and edit your essay.
Acing your economics essay is not just about receiving a good grade but also about honing valuable skills such as critical thinking, analysis, and effective communication. The ability to write a clear and concise economics essay is an essential skill for any student pursuing a career in economics or related fields. By practicing these techniques, you can master the art of writing a compelling and effective economics essay and achieve success in your academic and professional pursuits.
Understanding H2 Maths – Vectors
Vectors is one of the infamous topics that students find trouble with. The questions may seem difficult to approach, and most questions are split into parts, which means that most of the time students are required to complete the first part in order to complete the subsequent parts. However, with a good understanding of vectors and practice, vectors is not as difficult as it seems. In fact, most questions are repetitive as there is only so much that the exam can test.
UNDERSTANDING H2 CHEMISTRY – EQUILIBRIA
HANDY TIPS FOR H2 Chemistry – Equilibria
Are you struggling with inorganic chemistry and finding it difficult to calculate accurately during the exams? This article is here to highlight important concepts in Chemical Equilibria, which is the study of factors that disrupt the equilibrium, what happens when there is disequilibrium and how to bring the reaction back to dynamic equilibrium. This article will help you understand them better and provide you with some answering techniques to help you approach those questions better in your next examination.
Important concepts that are frequently tested:
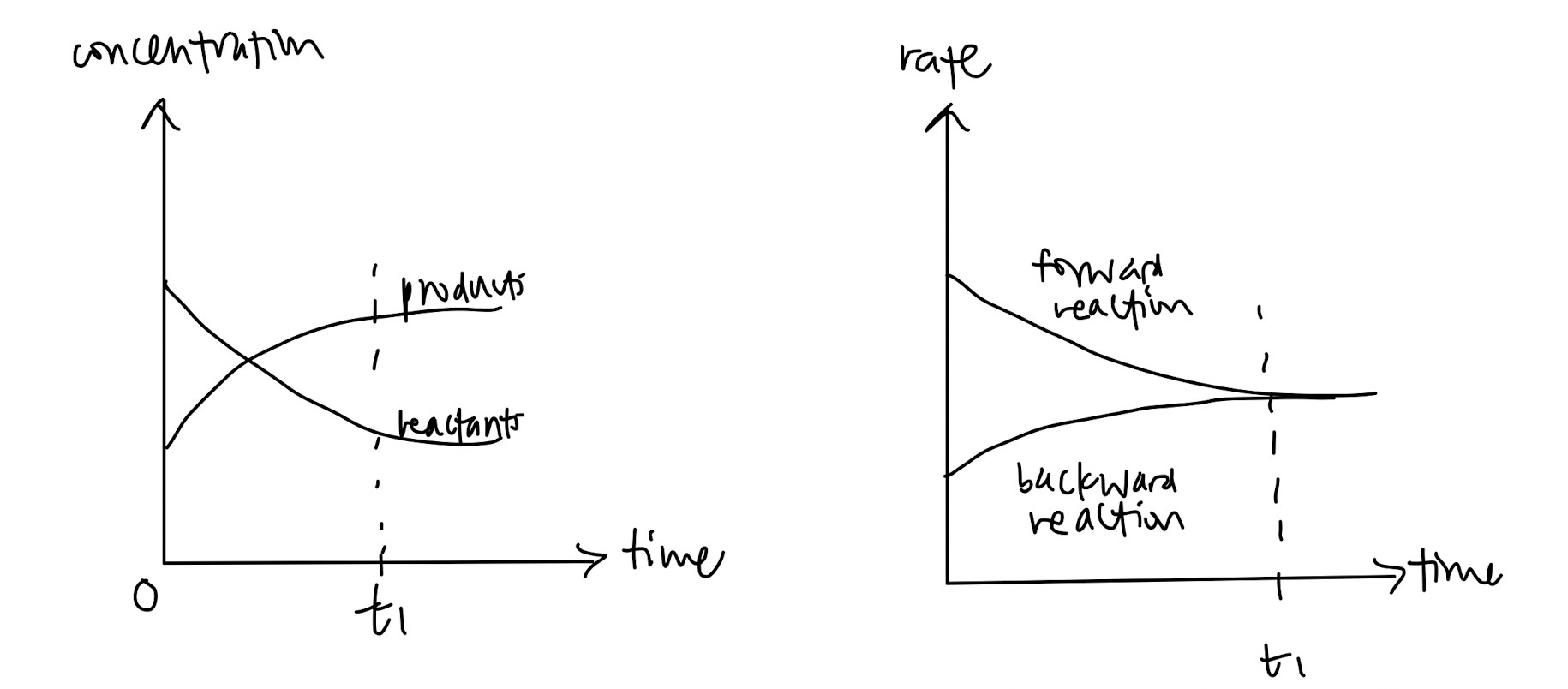
Definition of dynamic equilibrium – state of equilibrium where rate of forward reaction equals to rate of backward reaction (super easy to remember)
Knowing how to draw the concentration-time graph and rate-time graph
KC
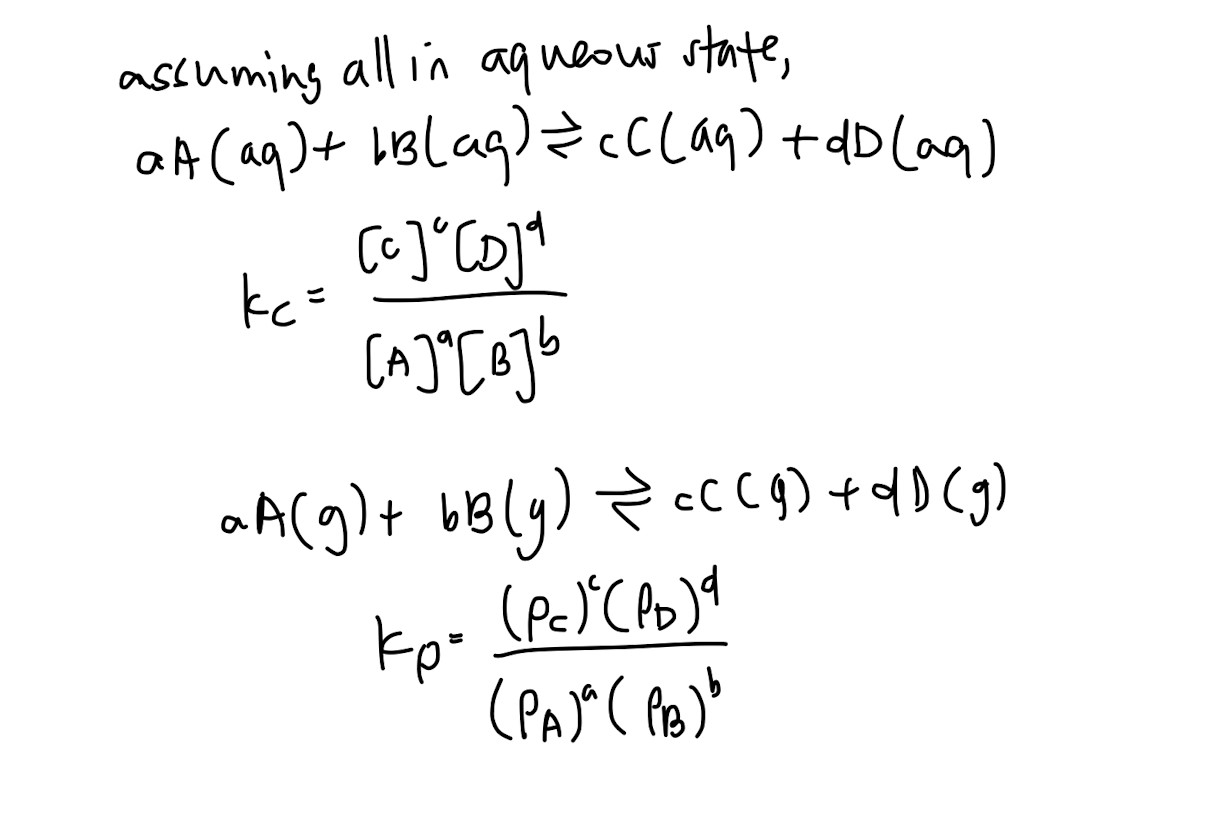
Kp (very similar to Kc)
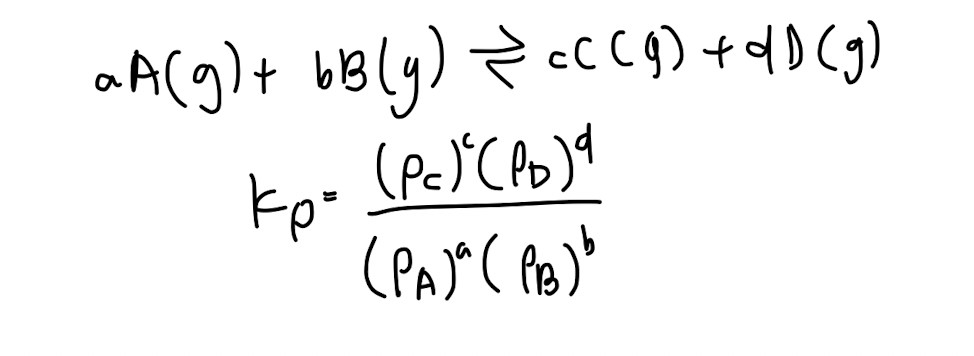
Kp and Kc are only affected by temperature (often tested to trick you)
Gibbs free energy: what does it mean when delta G < 0, delta G = 0, delta G > 0
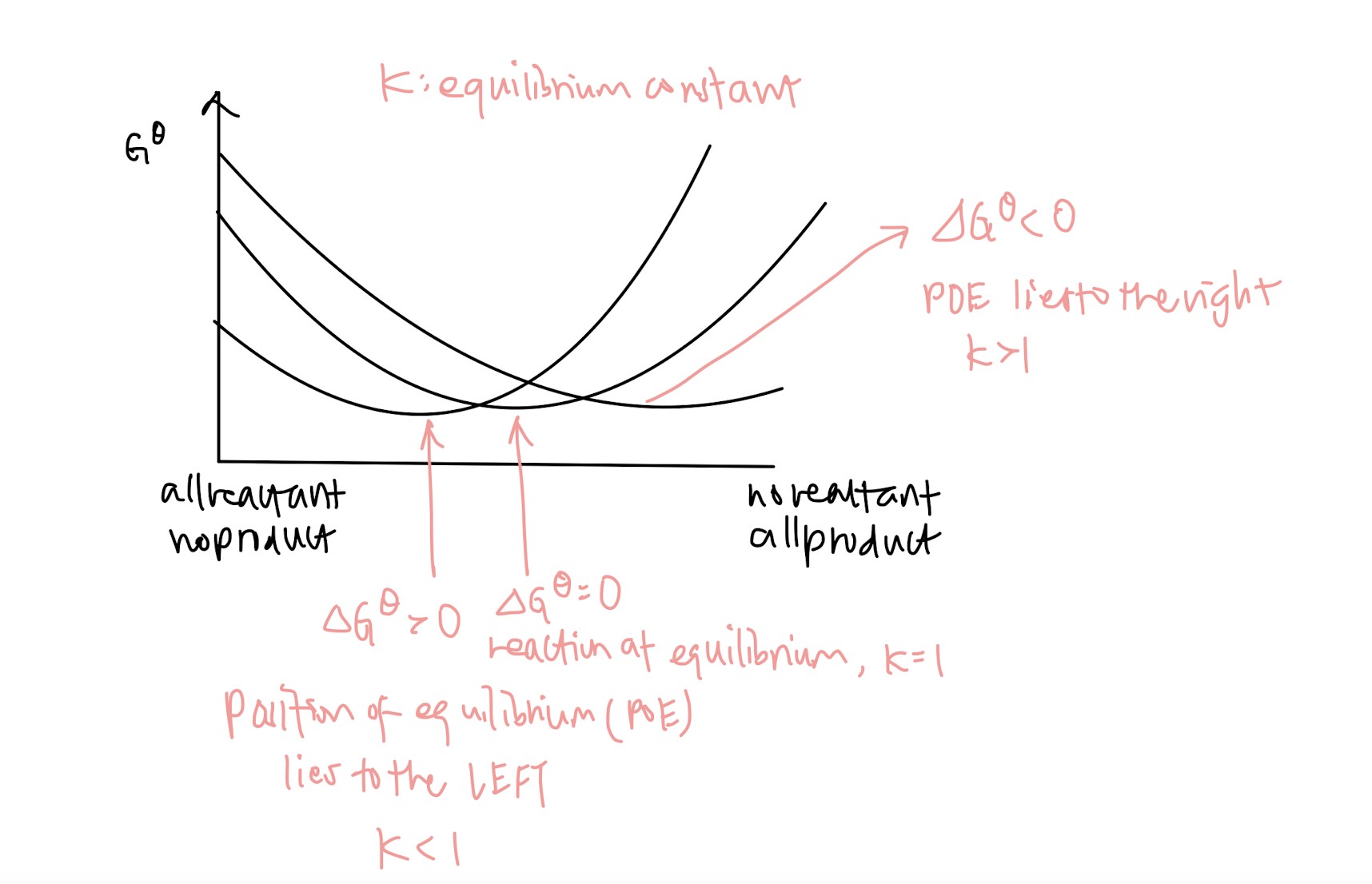
Definition of Le Chatelier’s Principle: If the conditions of a system in equilibrium are changed, the position of equilibrium moves to counteract that change (another easy mark)
How to draw the graph when concentration, temperature or pressure changes (Le Chatelier’s Principle is only affected by these three factors!!)
For example, if concentration increases,
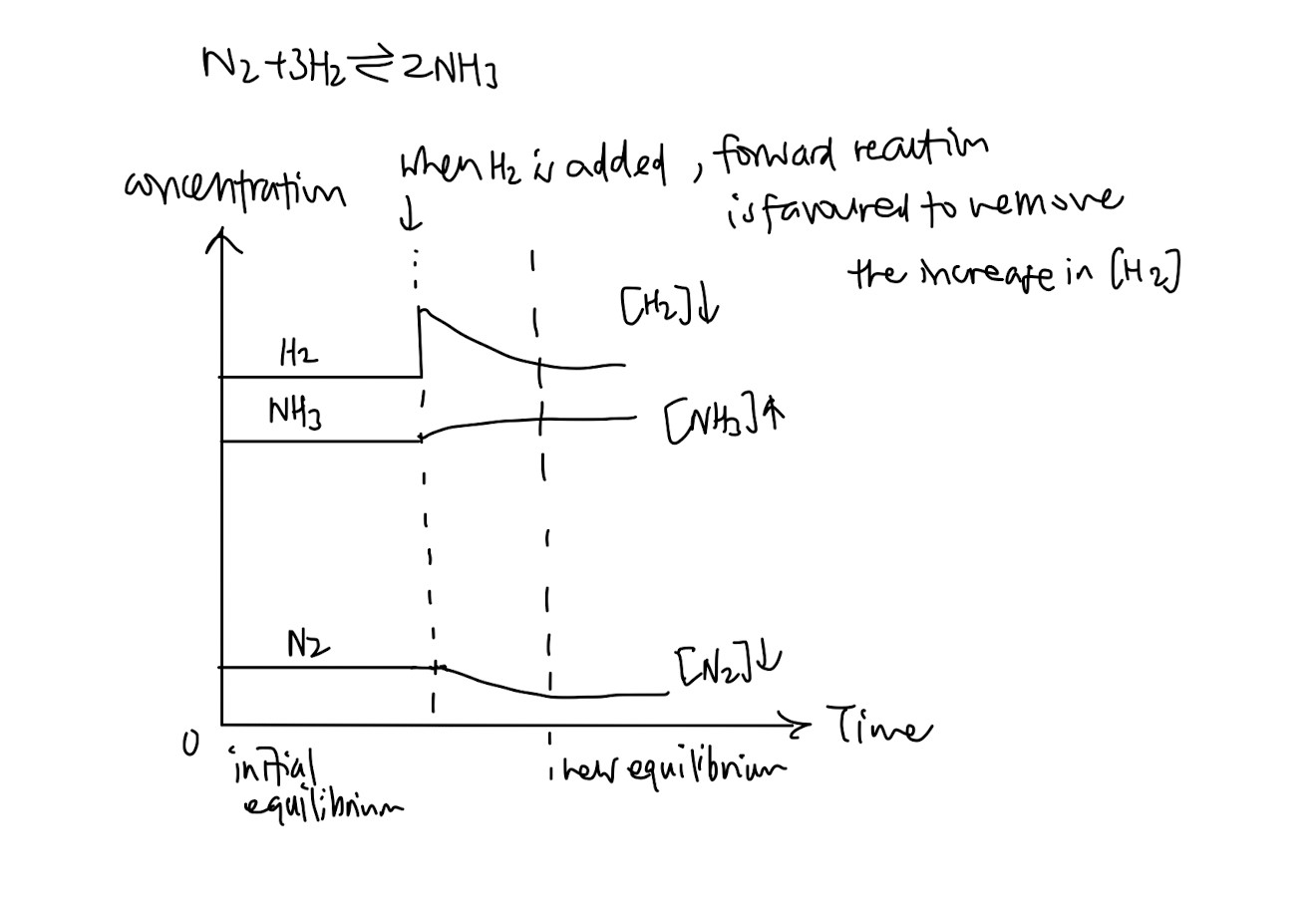
When in doubt, use the definition of Le Chatelier’s Principle to help you
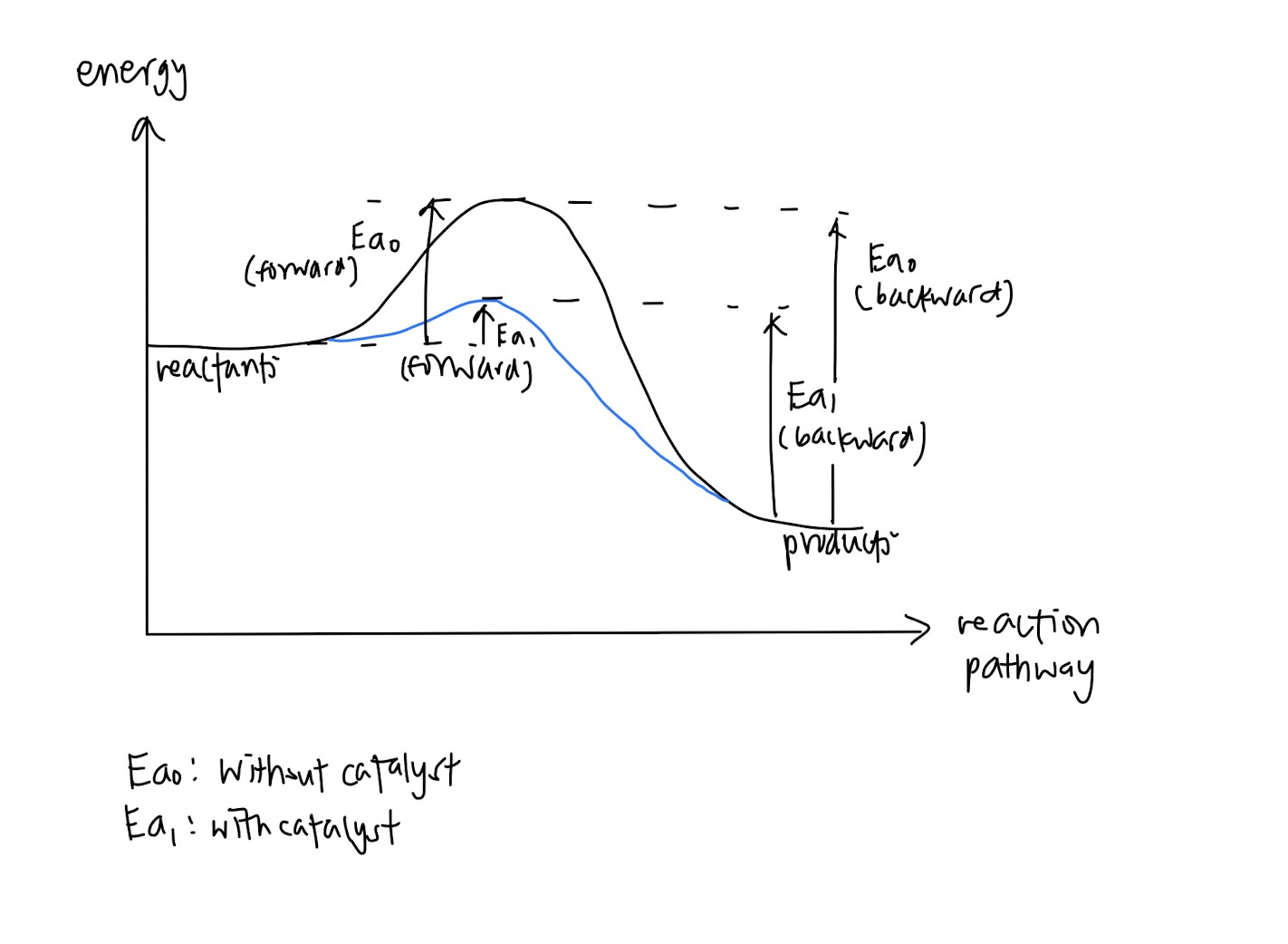
Effects of adding a catalyst
- Very simple, just remember catalyst is something that provides an alternative pathway with lower activation energy, and since less activation energy is needed, less time is needed for reaction to take place and rate of reaction decreases.
- This is not really tested but if they do, just remember that catalysts remain chemically unchanged
- Graph is important! Might be tested in MCQs or you may be asked to draw it out in paper 2 or 3
- Remember that catalyst never changes the position of equilibrium because they increase both the rate of forward reaction and backward reaction!! (often tested in MCQs) and since the catalyst does not change temperature, Kc does not change
Haber Process
- Remember the usual conditions for pressure and temperature (follow what your school suggests because different schools teach this differently)
- Catalyst is usually finely divided iron (because if iron pieces are too big, it will be less effective)
- Link it to the LCP:
- Pressure is high to favour forward reaction and increase yield but cannot be too high because that will require very strong pipes and vessels which will be more expensive
- Temperature is lower to favour forward reaction but cannot be too low either, or else the reaction will take very long and the process will not be cost-effective
Answering tips:
- When in doubt, always use the ICE table and you will be less likely to go wrong

- Remember to write the correct states of molecules in the equation!
Some concepts that may be harder to understand:
- When noble gas is added at constant pressure
- Use pV=nRT and partial pressure =
- When you add more molecules, in this case, noble gas molecules, total volume increases, but since the total pressure of the system stays constant, the proportion of total pressure with regards to each type of molecule decreases
- When noble gas is added at constant volume
- Use pV=nRT again
- When you add more molecules at constant volume, the total pressure increases (take the analogy of pumping more air into an enclosed container)
- However, since volume does not change, ratio of moles of each gas to the volume of the container will not change, hence partial pressure stays constant.
If you need any help with H2 inorganic chemistry or other chemistry topics, our professional and very experienced tutors are always available to help.
Make an enquiry here and our friendly education specialists will be in touch with you shortly.
Understanding H2 Chemistry – Energetics
HANDY TIPS FOR H2 Chemistry – Chemical Energetics
Are you struggling with inorganic chemistry and finding it difficult to calculate accurately during the exams? This article is here to highlight important concepts in Chemical Energetics, which is mainly on energy changes during reactions, such as the different standard enthalpy changes, and exothermic and endothermic reactions. We hope this article can help you understand them better and provide you with some answering techniques to help you approach those questions better in your next examination.
Important concepts that are frequently tested:
- Endothermic and exothermic – Definitions and how the graphs look like!
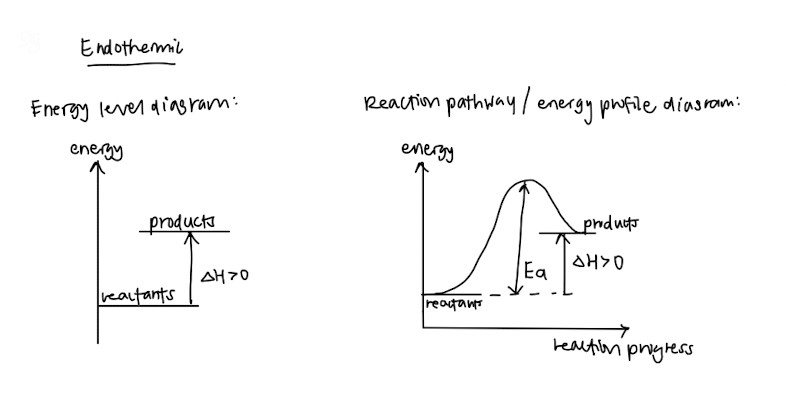
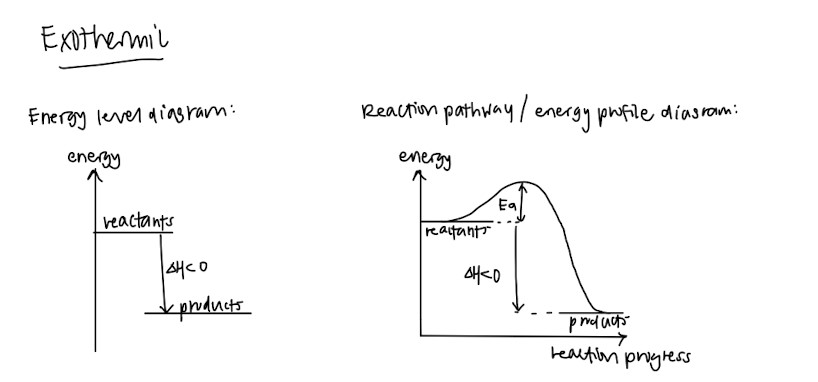
- Standard conditions and standard states (This should be standardised among schools but please double check) – They like to test this in MCQs or as a one mark question so please memorise these so you can get your free marks
– Temperature: 298K or 25 deg C
– Pressure: 1 bar = 10^5 Pa
– Concentration of any solution: 1.0 mol/dm3
– Standard states under standard conditions have zero enthalpy
Memorising the different enthalpy terms:
Of course, everything comes with practice, so don’t worry, the more you practise, the more you can commit these definitions to memory. To start off, remember most definitions end with the phrase “at 298K and 1 bar”. Substances are considered in the form of one mole, because you want to do calculations in its simplest form. The following list only includes the standard enthalpy changes that are frequently tested or confusing, but you should familiarise yourself with all the standard enthalpy changes in your syllabus.
Standard enthalpy change of formation
Simply put, the word formation makes remembering this definition very intuitive. Essentially, it is just the enthalpy change when 1 mole of substance is formed from its constituent elements in their standard states at 298K and 1 bar
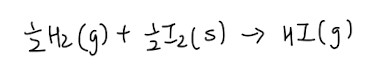
- By right, the enthalpy is supposed to be 0 kJ/mol
- If enthalpy is positive, compound is less stable
- If enthalpy is negative, compound is more stable
Standard enthalpy change of combustion
Burning always releases energy in the form of heat so the definition is heat evolved when 1 mole of substance is completely burnt in excess oxygen at 298K and 1 bar. You want combustion to be complete and in excess oxygen, otherwise other substances will be formed alongside.
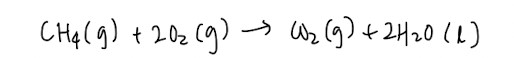
- Hess’ Law
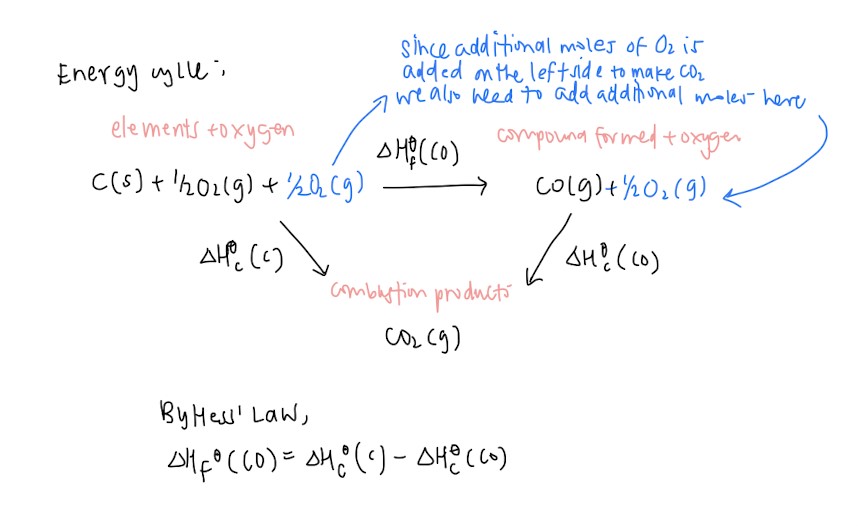
- Energy level diagram
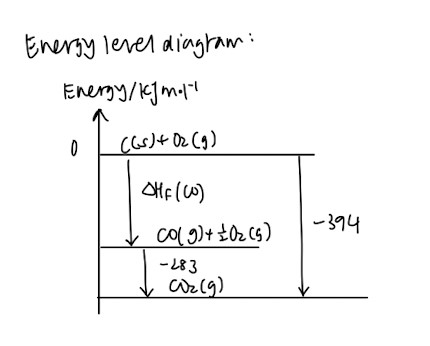
(Usually quite complicated at first, but it gets better when you familiarise yourself with the different definitions)
Bond dissociation energy
As the word “dissociation” suggests, you want to break something, and thus you need energy in order to do so, thus, bond dissociation will always be an endothermic process. As such, the definition is the energy required to break 1 mole of a particular covalent bond in a specific molecule in gaseous state.
- ΔH = sum of ΔH (bonds broken) – sum of ΔH (bonds formed)
- When calculating bond energies, remember to use the correct numbers. If you have multiple moles of a substance, you are supposed to multiply the bond energies by the number of moles of that substance (Students often lose marks here!)
Lattice Energy (LE)
It is the heat evolved when 1 mole of solid ionic compound is formed from its constituent gaseous ions. Since it is forming electrostatic forces of attraction between oppositely charged ions, LE always releases heat and is thus, always negative. Using this logic, the more negative LE is, the stronger the ionic bonding, and the more stable the ionic compound.
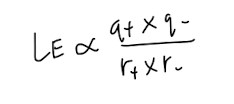
MCQs frequently test factors affecting lattice energy, so make sure you remember that and the equation for LE too! The factors affecting LE are:
- Ion charge
- Ion size/ size of inter-ionic distance
Ionisation Energy (IE)
First IE: energy required to remove 1 mole of electrons from 1 mole of gaseous atoms to form 1 mole of singly charged gaseous cations (The number of moles is very important, and remember to change the “singly charged” accordingly if the question is asking for second IE or third IE)
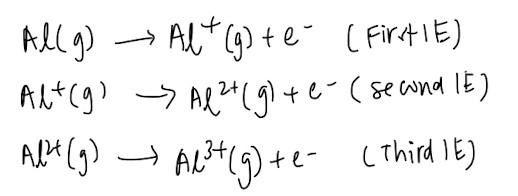
Electron Affinity (EA)
First EA: Enthalpy change when 1 mole of electrons is added to 1 mole of gaseous atoms to form 1 mole of singly charged gaseous anions
Relationship between LE, enthalpy changes of hydration and enthalpy changes of solution
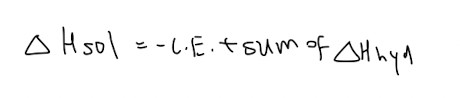
Gibbs Free Energy:
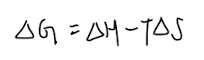
- When in doubt, use the equation to help you
- Remember the definition of entropy (measure of disorderliness/ randomness in the system)
- Factors that affect entropy
- Higher entropy: higher temperature, vaporisation/ melting, increase in gaseous particles, weaker intermolecular forces
- Lower entropy: dimerisation, water molecules hydrating ions are more orderly arranged than those in pure water
Very important concept that you should master: Born-Haber cycle (Energy level diagram especially)
- This is similar to the energy level diagram under the “Standard enthalpy change of combustion” section
- Make sure you look carefully at the number of moles involved in the reaction, because once you miscalculate the number of moles, you will calculate the LE wrongly and you will not get the full marks!
- Do more practices to familiarise yourself with the drawing of the energy level diagram and calculation
Questions with very standard answers:
- Why is there a higher experimental LE than theoretical: Higher experimental LE compensates for IE required
- What a large difference in experimental LE and theoretical LE mean: The ionic compound has covalent character.
- Why is 2nd IE higher than 1st IE: Further removal of electrons require more energy because shielding effect is reduced since there are less electrons.
- Why is 1st EA usually negative: The effective nuclear charge of the atom attracts the incoming electron. When there is a stronger attraction, the energy evolved is greater and LE is thus more negative.
- Why subsequent EA is always positive: Energy is required to overcome the electrostatic repulsion between the incoming electron and anion.
- Why a spontaneous reaction occurs at a slow rate or does not occur at all: Reaction requires high activation energy
- When the question asks why your calculated bond energy is different from the bond energy they give, remember the standard answer for this: Bond energy data in the data booklet are average values derived from a large range of molecules containing that particular covalent bond.
- Remember bond energy considers all molecules in gaseous state (common mistake among students)
Final words of advice
- Practice makes perfect! Keep practising energy-level diagram questions and calculations
- Don’t blindly memorise the standard enthalpy changes, try your best to understand the concept first before memorising them – it makes memorising a lot easier
If you need any help with H2 inorganic chemistry or other chemistry topics, our professional and very experienced tutors are always available to help.
Make an enquiry here and our friendly education specialists will be in touch with you shortly.
Understanding H2 Organic Chemistry – Part 2
Handy tips for H2 organic chemistry – Part 2
In part 1 of Learning H2 organic chemistry, you learn about concepts that are used in questions that are easy to score, including Hybridisations, Delocalisation energy, movement of electrons, and Degree of substitution. In this article, you will learn more about the other H2 organic chemistry concepts that usually come out during exam and are easy to score.
Some of the concepts include:
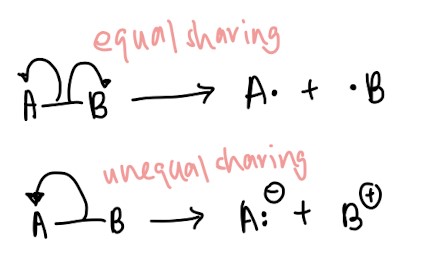
- Homolytic fission vs heterolytic fission: Use english to help you! Homo means same so homolytic fission means the pair of electrons is split evenly between two atoms. Use the same logic for heterolytic fission, hetero meaning different. ↓(Remember how to draw it!)↓
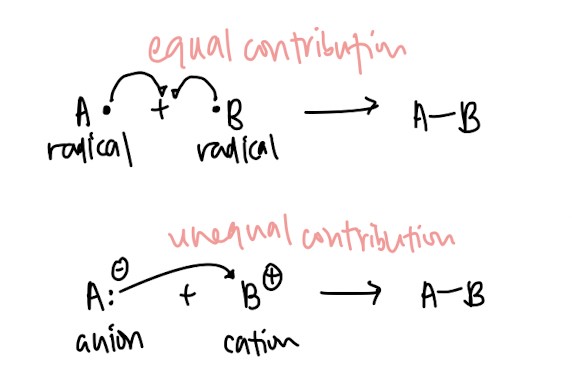
- Homolytic bond formation vs heterolytic bond formation: Same logic as above ↓(Remember how to illustrate it!)↓
- Free radical substitution: Memorise the mechanism and ↓how to draw it↓! It is commonly tested.
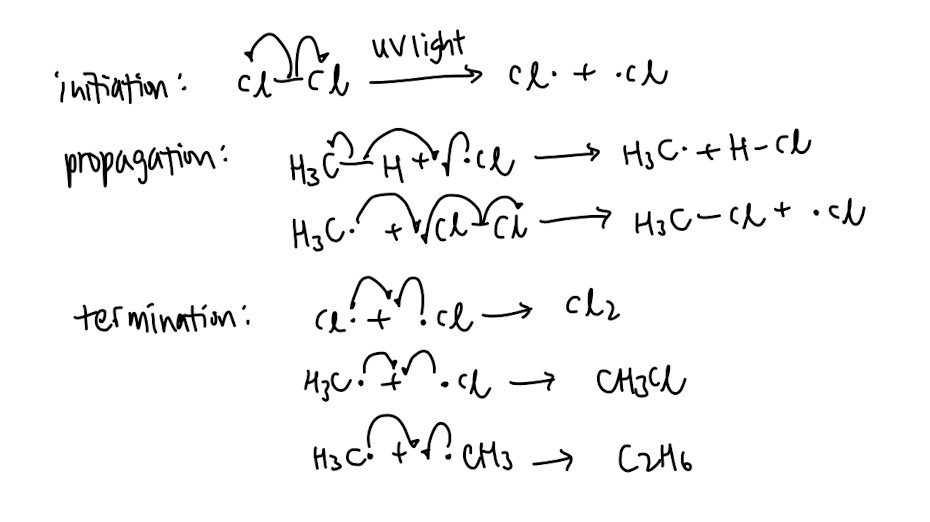
- Identifying and drawing stereoisomers, which are isomers that differ in the arrangement of atoms in space
- Aromatic compounds:
- Why aromatic compounds are only reactive towards electrophiles: The benzene ring is very electron-rich, thus it attracts electron deficient electrophiles/ repel electron-rich nucleophiles.
- Why aromatic compounds tend to undergo substitution: electrophilic addition disrupts aromaticity and destroys delocalisation; substitution keeps benzene ring unsaturated.
- Why OH- is used to generate a stronger nucleophile phenoxide ion: Because negative charge is more easily delocalised into benzene ring
- Why is there no electrophilic addition of the benzene ring: You want to maintain the aromatic structure and retain resonance stabilisation
Organic Nomenclature: Also an important concept because they may test you on naming but its not as hard as it seems. You don’t have to remember the order of functional group priorities entirely but just be aware of the prominent ones, and it will make more sense as you do more practices.
- If you see a carboxylic acid functional group, it will obviously become the suffix
- Parent nomenclature is the one with the longest main carbon chain
- Esters: The first part of the name is bound to the oxygen
Questions with very standard answers:
- Inductive effect
- Resonance effect (electron-withdrawing, electron-donating)
- Steric effects: Bulky alkyl group blocks path of electrophile
- Stereoisomerism: C=C cannot undergo free rotation unless pi bond is broken. The restricted rotation causes (compound) to be non-superimposable
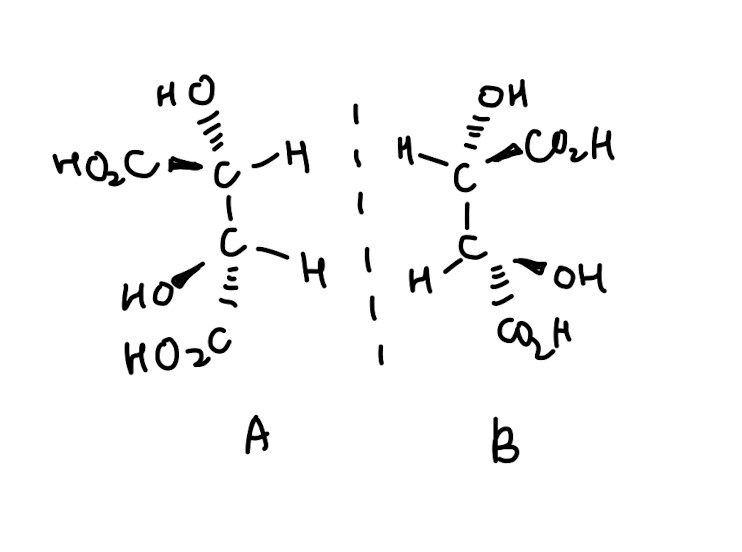
- Why are A and B able to rotate plane-polarised light: They are chiral and enantiomers, which each enantiomer being optically active and able to rotate plane polarised light in equal but opposite directions
Difference between electrophilic and nucleophilic:
“-phile” means being attracted to something so using that logic, electrophile attracts electrons while nucleophiles donate their pair of electrons to the electrophile.
Memorising types of reactions: Use logic and practise drawing the reactions to memorise them efficiently!
Final words of advice
- Do more practices! The more you do, the more you will be able to grasp those concepts and commit the keywords to memory. In fact, as you practise, you will realise that questions are usually repetitive and test on the same few concepts.
- When memorising the different equations, as well as how to draw them, pay close attention to patterns. Once you identify those patterns, it will be much easier to understand how each reaction work and remember them better!
If you need any help with H2 organic chemistry or other chemistry topics, our professional and very experienced tutors are always available to help.
Make an enquiry here and our friendly education specialists will be in touch with you shortly.
Understanding H2 Organic Chemistry – Part 1
Handy tips for H2 organic chemistry
Are you struggling with organic chemistry and finding it difficult to answer organic chemistry questions in the exams? Organic chemistry is about the study of structures, properties of and reactions between carbon-containing compounds and is not as daunting as it seems! This article is here to highlight important concepts in organic chemistry, help you understand them better and provide you with some answering techniques to help you approach those questions better in your next examination. Note that this article does not include specific equations for chemical reactions because those are rather straightforward and can be mastered if you put in the effort to memorise and practise them.
Concepts used in questions that are easy to score:
- Hybridisations
- Questions like these usually test your knowledge of sp, sp2 and sp3 orbitals by asking you to draw out the hybrid orbitals for a given molecule, eg. CH3
- To answer these questions, you need to familiarise yourself with the type of bonds (sigma or pi, single or double), the bond angle and bond shape for the different types of hybridisations
- sp3: As the name suggests, there are 1 s orbital and 3 p orbitals. By adding them together, you get 4 single bonds, and knowing that single bonds are only sigma bonds, sp3 hybridisations consist of 4 single sigma bonds and are used for molecules that only contain single bonds, for example, CH4.
- sp2: Using the same logic as above, there are 3 single bonds in this hybridisation. However, you know that one carbon atom needs to be share all 4 of their valence electrons and 3 single bonds only require the sharing of 3 electrons. Hence, one of the bonds must be a double bond. Knowing that double bonds are pi bonds, sp2 hybridisations thus consist of 3 sigma bonds and 1 pi bond, 3 single bonds and 1 double bond. sp2 hybridisations are used for molecules that contain only one double bond, for example, C2H4
- sp: Using the same logic, there are 2 single bonds, but in order for carbon to share all 4 valence electrons, you need 2 double bonds. However, it is chemically unstable to have C=C=C to accomodate 2 double bonds, thus, you can only have a triple bond. sp hybridisation are only used for molecules with one triple bond, for exam C2H2
- Using hybridisation to explain basicity: for example, sp2 – more s character, lone pair of electron is closer to the nucleus and hence, less available for donation
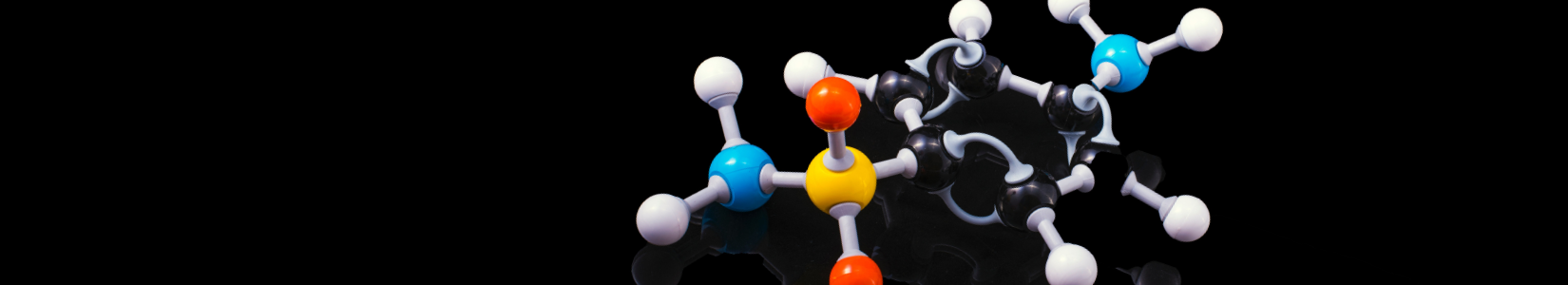
- Delocalisation energy: The more stable a compound is, the less negative ΔH(hydrogenation) is
- Formulae of organic compounds (very intuitive, just remember what each type of formula is) but remember to read the questions very carefully – some questions require you to use the displayed formula, skeletal formula or stereochemical formula, while some questions allow both.
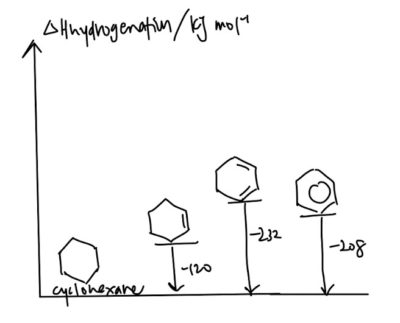
- Drawing movement of electrons: this is a super easy question to get marks easily from so don’t lose the opportunity!
- Full arrows (2 electrons): direction from anion (negative) to electropositive bonded atom – uses the logic unlike poles attract. Since the electropositive atom has been used to form bonds with the anion, the shared pair of electrons used to form the previous covalent automatically goes to the currently displaced atom (previously electronegative atom), forming an anion
- Half arrow (1 unpaired electron): In this case, electrons used for bonding with the unpaired electron come from the single bond.
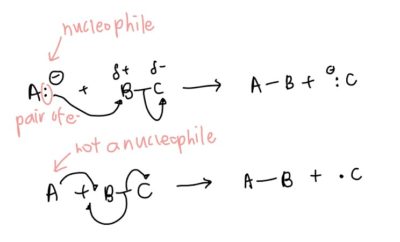
- Degree of substitution: another very straightforward concept
- Primary: One alkyl group bonded to main carbon atom
- Secondary: Two alkyl group bonded to main carbon atom
- Tertiary: Three alkyl group bonded to main carbon atom
- Quarternary: Four alkyl group bonded to main carbon atom
The next part will be covering the other 5 concepts that are easy to score, organic nomenclature, questions with very standard answers and some final words of advice.
If you need any help with topics such as H2 organic chemistry, Learners’ Lodge tutors are always available to help.
Make an enquiry here and our friendly education specialists will be in touch with you shortly.
Guide to doing well for JC Mathematics!
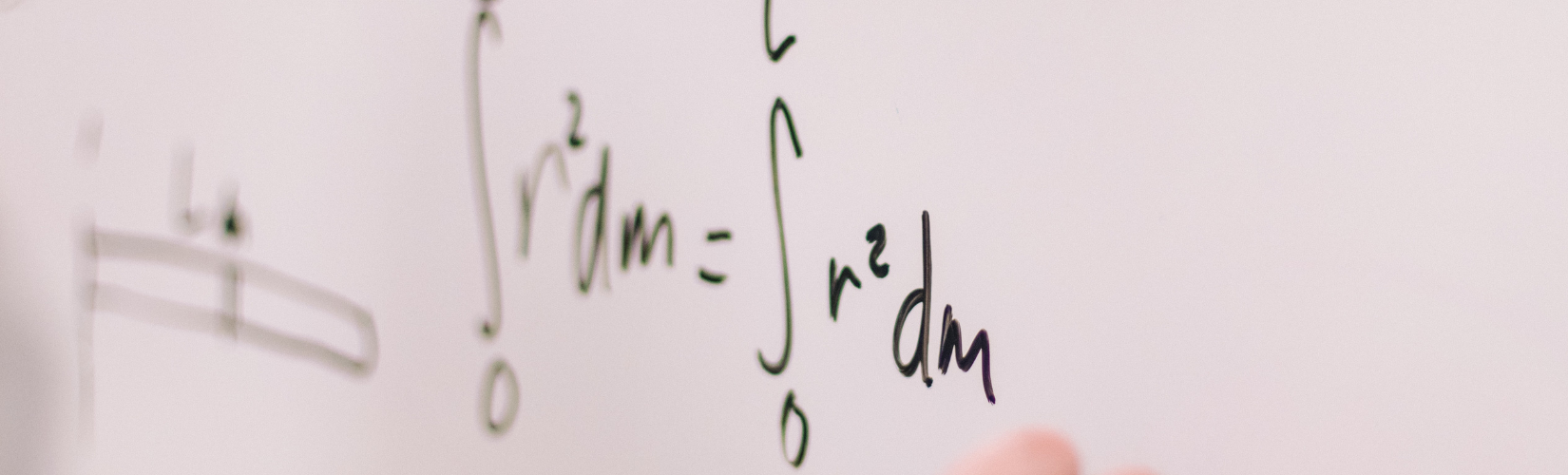
Guide to doing well for JC Mathematics!
By: Gabrielle Lee | Image: jeswin-thomas
Do you dislike Mathematics? Tried studying the textbook from start to finish but still can’t see significant improvements in your results? I was in the same shoes as yours before, but how did I manage to progress from constantly struggling to topping my class and scoring As at every Math examination?
Mathematics is actually not as scary, and as compared to other subjects, Mathematics is not as content-heavy! Formulas may be hard to memorise, because they seem so abstract, but you can quicken the memorising process if you keep doing practices. In essence, the key to acing Mathematics is to just keep practising!
Tip 1: Make summaries and use them
School notes may seem very daunting because they are thick, full of words, and quite messy at times. During my secondary school and JC days, I tackled this issue by writing all the important formulas, conditions and definitions into a simple notebook, and I would refer to it whenever I could not remember a formula, or for revision the day before the examination. The key here is not to make pretty notes, but to write simple and understandable notes, and to USE them!
Tip 2: Keep practising
Like what my math teacher used to say, it is actually much easier to see improvements in Math as compared to other subjects. All you need to do is to really commit time daily to practise! It’s just like playing a sport. You can’t seek improvements if you don’t put in effort to refine your skill.
Usually, after learning a new topic, I will use practice questions from the textbook or school worksheet to check if I understand the concept. Then, I will move on to extra questions that my school gave, for extra drilling. Afterwards, when it was nearer to the examinations, I will just keep doing questions from past year exam papers.
Tip 3: Review your mistakes!
Doing practice questions is completely useless, if you do not mark your work and do corrections. By marking your work, you have a gauge whether you actually understand the topic or not. However, that alone is insufficient. You need to do corrections to facilitate the process of understanding where you have gone wrong. It would be even better if you have the time to compare the differences between your answer and the model answer, and process what the lapses in your understanding are.
TIp 4: Look through your mistakes and challenging questions when you do your exam revision
It is always good to have a bank of challenging questions and questions you made mistakes it. However, if that is too troublesome, you can always flag out these questions using a highlighter or a post-it note. Afterwards, remember to look through these questions again before your exam so that you will not waste unnecessary marks by making the same mistakes as before.

Photo by Tony Tran on Unsplash
Mathematics is so boring! How do I push myself to spend more time studying it?
Mathematics may be tough for some, and since Mathematics is a compulsory subject, there are definitely some who may find no interest in it. However, my best advice will be to put in your best effort and try to enjoy the process. It’s easier said than done but you can try making Mathematics less dull for yourself by following the tips below!
Tip 1: Simplify the process
If you want to spend minimum time studying Mathematics, but get maximum results, you really need to be efficient. This means you should do away with things like making notes pretty, mult-tasking, and getting distracted. You can try setting aside 1 hour a day for you to study Mathematics without any external distractions and you will be surprised how much you can achieve during that one hour.
Tip 2: Start off with simpler questions
It’s definitely demoralising if you keep making mistakes and keep getting stuck at difficult questions. So, you can try gaining more confidence by starting off with simpler questions. As you progress, you will start to realise that Mathematics is actually rather doable and who knows, you may even start to enjoy it!
If you want to know more about how to do well in JC Maths, our friendly and professional teachers are here to guide you in acing Maths.
Click here to find out more.