H1 CHEMISTRY TUITION
(Syllabus 8873) 2019
H1 CHEMISTRY NOTES
Summary notes for H1 Chemistry will be provided for H1 chem tuition students of Learners’ Lodge so that you can study effectively and efficiently.
NEED HELP IN H1 CHEMISTRY?
H1 Chemistry candidates will be assumed to have knowledge and understanding of Chemistry at O Level, as a single subject or as part of a balanced science course. The H1 Chem syllabus is designed to place less emphasis on factual material and greater emphasis on the understanding and application of scientific concepts and principles. This approach has been adopted in recognition of the need for students to develop skills that will be of long term value in an increasingly technological world rather than focusing on large quantities of factual material which may have only short term relevance.
Experimental work is an important component and should underpin the teaching and learning of H1 Chemistry.



AIMS OF H1 CHEMISTRY
The aims of H1 Chemistry course based on MOE syllabus:
- Students develop interest in Chemistry and builds the knowledge, skills and attitudes necessary for them to become scientifically literate citizens well-prepared for 21st century challenges
- Students develop understanding, skills, ethics and attitudes relevant to the Practices of Science:
- Understanding nature of scientific knowledge
- Demonstrating science inquiry skills
- Relating science and society
- Students develop way of thinking to explain phenomena, approach and solve problems in chemical systems:
- Understanding the structure, properties and transformation of matter at the atomic/molecular level and how they are related to each other
- Connecting between the submicroscopic, macroscopic and symbolic levels of representations in explaining and making predictions about chemical systems, structures and properties.
Some students can find these learning aims and objectives too much to grasp on their own and would benefit from the guidance of a good H1 Chemistry tuition program. We have taken more than 10,000 students through to their A-levels! Try a class with us today.
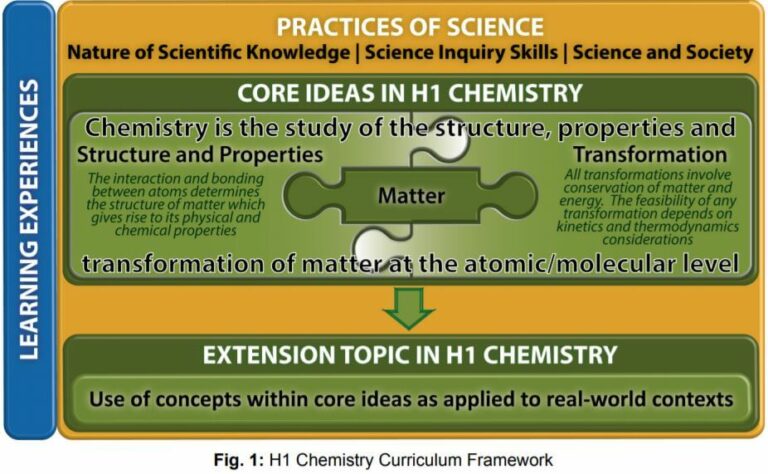
H1 CHEMISTRY CURRICULUM FRAMEWORK
The key features of the H1 Chemistry Curriculum comprise Core Ideas and Extension Topic, Practices of Science and Learning Experiences as illustrated in Fig. 1.
Core Ideas and Extension Topic for H1 Chemistry
The topics in the H1 Chemistry are organised as two levels underpinned by the Practices of Science:
- Core ideas: The three Core Ideas of Chemistry are Matter, Structure and Properties, and Transformation. The concepts in these Core Ideas are inter-related and form the basis for which further learning and understanding of chemical phenomena and reactions is built upon.
- Extension topic: Concepts in the Core Ideas are applied to real-world context in the study of nanomaterials and polymers.
Practices of Science for H1 Chemistry
Learning Experiences for H1 Chemistry
ASSESSMENT OBJECTIVES FOR H1 CHEMISTRY
The assessment objectives listed below reflect those parts of the Aims which will be assessed.
A. Knowledge with understanding
Students should be able to demonstrate knowledge with understanding in relation to:
- scientific phenomena, facts, laws, definitions, concepts, theories
- scientific vocabulary, terminology, conventions (including symbols, quantities and units)
- scientific instruments and apparatus, including techniques of operation and aspects of safety
- scientific quantities and their determination
- scientific and technological applications with their social, economic and environmental implications.
The Syllabus Content defines the factual knowledge that candidates may be required to recall and explain. Questions testing these objectives will often begin with one of the following words: define, state, describe, explain or outline. (See the Glossary of Terms)
B. Handling, applying and evaluating information
- locate, select, organise and present information from a variety of sources
- handle information, distinguishing the relevant from the extraneous
- manipulate numerical and other data and translate information from one form to another
- analyse and evaluate information so as to identify patterns, report trends and conclusions, and draw inferences
- present reasoned explanations for phenomena, patterns and relationships
- apply knowledge, including principles, to novel situations
- bring together knowledge, principles, concepts and skills from different areas of chemistry, and apply them in a particular context
- evaluate information and hypotheses
- construct arguments to support hypotheses or to justify a course of action
- demonstrate an awareness of the limitations of Chemistry theories and models.
These Assessment Objectives cannot be precisely specified in the syllabus content because questions testing such skills may be based on information which is unfamiliar to the candidate. In answering such questions, candidates are required to use principles and concepts that are within the syllabus and apply them in a logical, reasoned or deductive manner to a novel situation. Questions testing these objectives will often begin with one of the following words: predict, suggest, construct, calculate or determine (see the Glossary of Terms).
SCHEME OF ASSESSMENT FOR H1 CHEMISTRY
All candidates are required to enter for Papers 1 and 2.
| Paper | Type of Paper | Duration | Marks | Weighting(%) |
|---|---|---|---|---|
| 1 | Multiple Choice | 1 hour | 30 | 33 |
| 2 | Structure and Free Response Questions | 2 hours | 80 | 67 |
Paper 1 (1 h, 30 marks)
This paper consists of 30 compulsory multiple choice questions. Four to six items will be of the multiple completion type. All questions will include 4 options.
Paper 2 (2 h, 80 marks)
This paper consists of two sections. All answers will be written in spaces provided on the Question Paper.
Section A (60 marks)
A variable number of structured questions including data-based questions, all compulsory. The data-based question(s) constitute(s) 15–20 marks for this paper. The data-based question(s) provide(s) a good opportunity to test higher order thinking skills such as handling, applying, and evaluating information.
Section B (20 marks)
Candidates will be required to answer one out of two questions. Each question will carry 20 marks.
These questions will require candidates to integrate knowledge and understanding from different areas and topics of the chemistry syllabus.
MARKS ALLOCATED TO H1 CHEM ASSESSMENT OBJECTIVES
| Assessment Objectives | Weighting (%) | Assessment Components | |
|---|---|---|---|
| A | Knowledge with understanding | 40 | Papers 1, 2 |
| B | Handling, applying and evaluating information | 60 | Papers 1, 2 |
Data Booklet
A Data Booklet is available for use in the theory papers. The booklet is reprinted at the end of this syllabus document.
Nomenclature
Candidates will be expected to be familiar with the nomenclature used in the syllabus. The proposals in “Signs, Symbols and Systematics” (The Association for Science Education Companion to 16-19 Science, 2000) will generally be adopted although the traditional names sulfate, sulfite, nitrate, nitrite, sulfurous and nitrous acids will be used in question papers. Sulfur (and all compounds of sulfur) will be spelt with f (not with ph) in question papers, however candidates can use either spelling in their answers.
Units and significant figures
Candidates should be aware that misuse of units and/or significant figures, i.e. failure to quote units where necessary, the inclusion of units in quantities defined as ratios or quoting answers to an inappropriate number of significant figures, is liable to be penalised.
Disallowed Subject Combinations
Candidates may not simultaneously offer Chemistry at H1 and H2 levels.
For JC Chemistry Tuition Schedule and Tutors, please kindly visit the JC Chemistry Page.

You can register personally at any one of our branches during our operating hours. Alternatively, you may call our hotline 9119-9655 to register. Please note that phone registration does not constitute a reservation of a seat in the program. Only upon payment for the program will the seat(s) be reserved.
Looking for information on H2 Chem tuition instead?